Objective 6:
1. acid: something that reacts with a base to neutralizes its properties, something that tastes sour, something that reacts with an active metal to liberate hydrogen gas, and something that conducts electricity
2. neutralization: a chemical reaction when an acid and a base react to form salt
3. indicator: a halochromic (when the pH changes, the color also changes) chemical compound that you can add to a solution little by little to see the pH of a solution can be indicated visually
4. corrosive: if something is corrosive, then it will destroy or severely damage another thing when it comes into contact with it
5. hydroxide ion: the negatively charged ion in a water solution of a base (they type of ions that are formed when bases are dissolved in water)
Cites:
http://www.zellescienceblog.blogspot.com/
http://en.wikipedia.org/wiki/Neutralization_(chemistry)
http://en.wikipedia.org/wiki/PH_indicator
http://en.wikipedia.org/wiki/Corrosive_substance
http://www.yourdictionary.com/hydroxide-ion
Sunday, November 28, 2010
Test 10 ~ Objective 5
Objective 5:
1. The two parts of digestion are mechanical and chemical.
2. Mechanical digestion requires "manual" labor, like by chewing. Chemical digestion is when there is hydrochloric acid in your stomach and small intestine.
3. Well, if someone can't chew their own food, then their body can't preform mechanical digestion, so their body has to preform a lot more chemical digestion, which could maybe be overworked.
4. The pH in your mouth is 7. The pH in your stomach is 1.5 . The pH in your small intestine is 8.
5. Different enzymes work better with different pH values, and enzymes evolved in digestion can effect reactions within and outside of cells.
Cites:
www.mnsu.edu/emuseum/biology/.../digestive/index.shtml
http://wiki.answers.com/Q/What_is_the_difference_between_mechanical_digestion_and_chemical_digestion#ixzz16btmgJUm
http://wiki.answers.com/Q/What_is_the_ph_in_your_mouth
http://wiki.answers.com/Q/What_is_the_pH_of_the_stomach
http://wiki.answers.com/Q/What_is_the_ph_of_your_small_intestine
http://wiki.answers.com/Q/How_is_pH_important_during_digestion
1. The two parts of digestion are mechanical and chemical.
2. Mechanical digestion requires "manual" labor, like by chewing. Chemical digestion is when there is hydrochloric acid in your stomach and small intestine.
3. Well, if someone can't chew their own food, then their body can't preform mechanical digestion, so their body has to preform a lot more chemical digestion, which could maybe be overworked.
4. The pH in your mouth is 7. The pH in your stomach is 1.5 . The pH in your small intestine is 8.
5. Different enzymes work better with different pH values, and enzymes evolved in digestion can effect reactions within and outside of cells.
Cites:
www.mnsu.edu/emuseum/biology/.../digestive/index.shtml
http://wiki.answers.com/Q/What_is_the_difference_between_mechanical_digestion_and_chemical_digestion#ixzz16btmgJUm
http://wiki.answers.com/Q/What_is_the_ph_in_your_mouth
http://wiki.answers.com/Q/What_is_the_pH_of_the_stomach
http://wiki.answers.com/Q/What_is_the_ph_of_your_small_intestine
http://wiki.answers.com/Q/How_is_pH_important_during_digestion
Test 10 ~ Objective 4
Objective 4:
1. The common ion found in all acids is H+.
2. When acids and bases dissolve in water, acids form hydrogen ions (hydrogens that have lost their electron), and bases form hydroxide ions.
3. The ions that the acid NHO3 will form after being dissolved in water are H + NO3.
4. A substance's pH tells you the amount of H+ ions there are in it. If the pH is higher lower that 7, it is an acid, and if it is higher, it is a base.
5. When the pH is low, the concentration of hydrogen is higher.... so the pH of 3 would have more hydrogen ions. Basically, when the pH is lower than 7, there are more hydrogen ions that hydroxide ions (because anything with a pH lower than 7 is an acid, and when acids dissolve in water, they form hydrogen ions... so there are more.) Similarly, when the pH is higher than 7, there are more hydroxide ions than hydrogen ions for the same reasons.
Cites:
http://wiki.answers.com/Q/What_ion_is_present_in_all_acidic_solutions
http://wiki.answers.com/Q/What_happens_when_acids_and_bases_dissolve_in_water
http://answers.yahoo.com/question/index?qid=20080108190009AA0Uied
http://answers.yahoo.com/question/index?qid=20090102141813AAr1kRp
http://wiki.answers.com/Q/What_solution_has_a_greater_concentration_of_hydrogen_ions_a_solution_with_a_pH_of_3_of_a_pH_of_7_Explain
1. The common ion found in all acids is H+.
2. When acids and bases dissolve in water, acids form hydrogen ions (hydrogens that have lost their electron), and bases form hydroxide ions.
3. The ions that the acid NHO3 will form after being dissolved in water are H + NO3.
4. A substance's pH tells you the amount of H+ ions there are in it. If the pH is higher lower that 7, it is an acid, and if it is higher, it is a base.
5. When the pH is low, the concentration of hydrogen is higher.... so the pH of 3 would have more hydrogen ions. Basically, when the pH is lower than 7, there are more hydrogen ions that hydroxide ions (because anything with a pH lower than 7 is an acid, and when acids dissolve in water, they form hydrogen ions... so there are more.) Similarly, when the pH is higher than 7, there are more hydroxide ions than hydrogen ions for the same reasons.
Cites:
http://wiki.answers.com/Q/What_ion_is_present_in_all_acidic_solutions
http://wiki.answers.com/Q/What_happens_when_acids_and_bases_dissolve_in_water
http://answers.yahoo.com/question/index?qid=20080108190009AA0Uied
http://answers.yahoo.com/question/index?qid=20090102141813AAr1kRp
http://wiki.answers.com/Q/What_solution_has_a_greater_concentration_of_hydrogen_ions_a_solution_with_a_pH_of_3_of_a_pH_of_7_Explain
Test 10 ~ Objective 3
Objective 3:
1. The four properties of acids are that they are sour tasting, conduct electricity, they react to a base to neutralize its properties, and acts reacts with active metals to liberate hydrogen gas.
1. The four properties of acids are that they are sour tasting, conduct electricity, they react to a base to neutralize its properties, and acts reacts with active metals to liberate hydrogen gas.
2. The four properties of bases are that they are bitter tasting, conduct electricity, they react to acids to neutralized its properties, and feels slippery to the skin
3. You can use the litmus paper by pouring the acid or base onto it, and you can see wether it is an acid or a base by what color the paper turns. If it turns red, it is an acid, and if the paper turns blue, it is a base.
4. You can tell if a food contains acid because it will taste sour (like I said in number one.) An example of a drink that contains acid is orange juice, and it can be very sour.
5. It is important to wear gloves when you are spreading fertilizer in the garden because there are chemicals that will burn or irritate your hands. Maybe there are acids in the fertilizer that could burn you.
Cites:
Friday, November 26, 2010
Test 10 ~ Objective 2
Objective 2:
1. Concentration is the measure of how much of another substance is in a different substance. To measure, you need to know the ratio of the solvent to the solute.
2. Solubility can be useful in finding the substances because it is a characteristic of common matter. The common characteristic of solubility can be used to figure out how much of a certain substance can be dissolved in the solvent. So, if you know the solubility, then you know how much of a solute can be added to the solvent until it is at its maximum concentration, which I talked about in the first question.
3. The three factors that affect solubility are temperature, pressure, and polarity.
4. Heat is needed to break the bonds that holds the molecules together, and solubility depends on heat.
5. Solubility is helpful in seeing the substances because you can see how much of something is able to be dissolved in it.
Cites:
http://en.wikipedia.org/wiki/Concentration
http://wiki.answers.com/Q/Why_is_solubility_useful_in_identifying_substances
http://en.wikipedia.org/wiki/Solubility
http://www.solubilityofthings.com/basics/factors_affecting_solubility.php
http://en.wikipedia.org/wiki/Solubility#Temperature
1. Concentration is the measure of how much of another substance is in a different substance. To measure, you need to know the ratio of the solvent to the solute.
2. Solubility can be useful in finding the substances because it is a characteristic of common matter. The common characteristic of solubility can be used to figure out how much of a certain substance can be dissolved in the solvent. So, if you know the solubility, then you know how much of a solute can be added to the solvent until it is at its maximum concentration, which I talked about in the first question.
3. The three factors that affect solubility are temperature, pressure, and polarity.
4. Heat is needed to break the bonds that holds the molecules together, and solubility depends on heat.
5. Solubility is helpful in seeing the substances because you can see how much of something is able to be dissolved in it.
Cites:
http://en.wikipedia.org/wiki/Concentration
http://wiki.answers.com/Q/Why_is_solubility_useful_in_identifying_substances
http://en.wikipedia.org/wiki/Solubility
http://www.solubilityofthings.com/basics/factors_affecting_solubility.php
http://en.wikipedia.org/wiki/Solubility#Temperature
Tuesday, November 23, 2010
Test 10 ~ Objective 1
Objective 1:
1. In a solution, the solute particles have much smaller molecules than colloids and suspensions. It is a "homogeneous mixture" (is uniform in composition) of more than on substance. A few examples are salt, water, and sugar. Colloids are dispersed evenly throughout a substance. An example of this is milk. Suspensions are also homogeneous fluids that are too big for sedimentation.
2. When a solution is formed, the particles of the solute leave each other and become part of the solvent.
3. The solute lowers the freezing point of water because it is applied to a colligative property, which means that it depends on the number of dissolved particles in a substance, not the actual identity of the solutes (an example is the freezing point of salt water is lower than the freezing point of water because the salt dissolves into the water.) It will raise the boiling point for the same reason.
4. You would make a solution because the food coloring is obviously UNIFORMLY spreading through the water to make it blue.
5. The solute lowers the freezing point of water because it is applied to a colligative property, which means that it depends on the number of dissolved particles in a substance, not the actual identity of the solutes (an example is the freezing point of salt water is lower than the freezing point of water because the salt dissolves into the water.) It will raise the boiling point for the same reason.
Cites:
http://www.infoplease.com/ce6/sci/A0861175.html
http://en.wikipedia.org/wiki/Solutions
http://en.wikipedia.org/wiki/Colloids
http://en.wikipedia.org/wiki/suspensions
http://wiki.answers.com/Q/What_happens_to_the_solute_particles_when_a_solution_forms
http://wiki.answers.com/Q/You_mix_food_coloring_in_water_to_make_it_blue_haveyou_made_a_solution_or_a_suspension
http://www.chemistryexplained.com/Ce-Co/Colligative-Properties.html
1. In a solution, the solute particles have much smaller molecules than colloids and suspensions. It is a "homogeneous mixture" (is uniform in composition) of more than on substance. A few examples are salt, water, and sugar. Colloids are dispersed evenly throughout a substance. An example of this is milk. Suspensions are also homogeneous fluids that are too big for sedimentation.
2. When a solution is formed, the particles of the solute leave each other and become part of the solvent.
3. The solute lowers the freezing point of water because it is applied to a colligative property, which means that it depends on the number of dissolved particles in a substance, not the actual identity of the solutes (an example is the freezing point of salt water is lower than the freezing point of water because the salt dissolves into the water.) It will raise the boiling point for the same reason.
4. You would make a solution because the food coloring is obviously UNIFORMLY spreading through the water to make it blue.
5. The solute lowers the freezing point of water because it is applied to a colligative property, which means that it depends on the number of dissolved particles in a substance, not the actual identity of the solutes (an example is the freezing point of salt water is lower than the freezing point of water because the salt dissolves into the water.) It will raise the boiling point for the same reason.
Cites:
http://www.infoplease.com/ce6/sci/A0861175.html
http://en.wikipedia.org/wiki/Solutions
http://en.wikipedia.org/wiki/Colloids
http://en.wikipedia.org/wiki/suspensions
http://wiki.answers.com/Q/What_happens_to_the_solute_particles_when_a_solution_forms
http://wiki.answers.com/Q/You_mix_food_coloring_in_water_to_make_it_blue_haveyou_made_a_solution_or_a_suspension
http://www.chemistryexplained.com/Ce-Co/Colligative-Properties.html
Wednesday, November 17, 2010
Test 9 ~ Objective 5
Objective 5:
1. Heat engines use thermal energy by expanding the gas. It causes the pistol to move down, so it lights the spark plug. They also turn it into mechanical energy.
2. Internal combustion engines are when the burning (combustion) of the fuel happens when the air gets into a combustion chamber. They use radiation for thermal energy. External combustion engines use the external heat to "energize" an internal fluid. These two are alike because they both use heat to run.
3. I think that modern cars use the internal combustion engines because these engines use a very common resource (air) to run, so it costs less money.
4. Unlike what we think, the refrigerator takes out all of the hot air, rather than putting cold air inside. This causes it to be cold.
5. A compressor in a refrigerator is the motor to the cooling system. This usually only runs when the thermostat of the refrigerator tells it to. If there was no compressor, all of the food would get hot because it wouldn't run when the thermostat told it to.
Cites:
http://www.ehow.com/how-does_4926292_car-engine-piston-work.html http://wiki.answers.com/Q/In_a_heat_engine_what_happens_to_thermal_energy_that_is_converted_from_chemical_energy
http://en.wikipedia.org/wiki/Heat_engine
http://www.alternatefuelsworld.com/heat-engines-part-3.html
http://en.wikipedia.org/wiki/Internal_combustion_engine
http://www.repairclinic.com/Refrigerator-How-Things-Work?red=#acompressor
1. Heat engines use thermal energy by expanding the gas. It causes the pistol to move down, so it lights the spark plug. They also turn it into mechanical energy.
2. Internal combustion engines are when the burning (combustion) of the fuel happens when the air gets into a combustion chamber. They use radiation for thermal energy. External combustion engines use the external heat to "energize" an internal fluid. These two are alike because they both use heat to run.
3. I think that modern cars use the internal combustion engines because these engines use a very common resource (air) to run, so it costs less money.
4. Unlike what we think, the refrigerator takes out all of the hot air, rather than putting cold air inside. This causes it to be cold.
5. A compressor in a refrigerator is the motor to the cooling system. This usually only runs when the thermostat of the refrigerator tells it to. If there was no compressor, all of the food would get hot because it wouldn't run when the thermostat told it to.
Cites:
http://www.ehow.com/how-does_4926292_car-engine-piston-work.html http://wiki.answers.com/Q/In_a_heat_engine_what_happens_to_thermal_energy_that_is_converted_from_chemical_energy
http://en.wikipedia.org/wiki/Heat_engine
http://www.alternatefuelsworld.com/heat-engines-part-3.html
http://en.wikipedia.org/wiki/Internal_combustion_engine
http://www.repairclinic.com/Refrigerator-How-Things-Work?red=#acompressor
Test 9 ~ Objective 4
Objective 4:
1. Temperature causes matter to change state (if it warms up, it gets closer to a gas, and if it gets cooler, it gets closer to a solid.)
2. As thermal energy increases, the matter begins to eat up. If it is a solid, it becomes a liquid. If it is a liquid, then it becomes a gas.
3. The temperature of matter stays the same because all of the temperature's energy is being put into the matter because it is changing its' state.
4. A solid melts because of the thermal energy acting on it. It causes the molecules to heat up and to start bouncing around. It basically expands and then melts away.
5. You have to poke holes in potatoes because they have so much water inside of them. When it gets really hot, it turns into steam. If the steam doesn't have a way out, it might explode.... so you poke holes in it to prevent it from exploding.
Cites:
Monday, November 15, 2010
Test 9 ~ Objective 3
Objective 3:
1. The three types of heat transfer are conduction, convection, and radiation.
2. Heat moves from places where there is a lot of heat to places where there isn't a lot of heat (from high concentration to low concentration.)
3. Conductors make it easier for energy to pass through them, and insulators (most solid matters) make it harder for energy to go through them.
4. A copper pipe would work better as a conductor because it has a lot of free electrons that can make the energy flow quicker and more efficient. Their electrons are in a cloud, so they aren't tightly packed.
5. If I were in the forest, I would build a fire instead of putting up a tent. If I made a tent, that would be conduction (atoms are in close proximity). This would help me because I would be insulated, which can be, in some ways, the best form of heat. If I built a fire (which I would), then that would be radiation (comes from the sun and travels in waves). The fire would give off warm air, which would help to keep me warm.
Cites:
http://www.tapinsulation.com/heat.html
http://en.wikipedia.org/wiki/Heat_transfer
http://hyperphysics.phy-astr.gsu.edu/hbase/electric/conins.html
http://www.highsierraadventures.com/skills/survival.htm
1. The three types of heat transfer are conduction, convection, and radiation.
2. Heat moves from places where there is a lot of heat to places where there isn't a lot of heat (from high concentration to low concentration.)
3. Conductors make it easier for energy to pass through them, and insulators (most solid matters) make it harder for energy to go through them.
4. A copper pipe would work better as a conductor because it has a lot of free electrons that can make the energy flow quicker and more efficient. Their electrons are in a cloud, so they aren't tightly packed.
5. If I were in the forest, I would build a fire instead of putting up a tent. If I made a tent, that would be conduction (atoms are in close proximity). This would help me because I would be insulated, which can be, in some ways, the best form of heat. If I built a fire (which I would), then that would be radiation (comes from the sun and travels in waves). The fire would give off warm air, which would help to keep me warm.
Cites:
http://www.tapinsulation.com/heat.html
http://en.wikipedia.org/wiki/Heat_transfer
http://hyperphysics.phy-astr.gsu.edu/hbase/electric/conins.html
http://www.highsierraadventures.com/skills/survival.htm
Sunday, November 14, 2010
Test 9 ~ Objective 2
Objective 2:
1. Thermometers measure temperature by telling how fast the particles inside of the object are moving. Thermometers use mercury, which expands when it gets hot and gets smaller when it cools down.
2. Kelvin and Celsius scales are the same because, although they have different zero points, they still have the same intervals, or degrees. They are different because they are on different scales (288 degrees Kelvin = 15 degrees Celsius = 59 degrees Fahrenheit.) Also, Kelvin isn't really a "degree" of temperature, like Fahrenheit and Celsius.
3. 5 degrees Celsius = 41 degrees Fahrenheit
4. 860 degrees Fahrenheit = 460 degrees Celsius
5. The temperature needs to be raised by 209,000 K.
Cites:
http://wiki.answers.com/Q/How_do_thermometers_measure_temperature
http://en.wikipedia.org/wiki/Temperature_measurement#Comparison_of_temperature_scales
http://wiki.answers.com/Q/How_are_the_three_temperature_scales_alike
http://geography.about.com/c/ht/00/07/How_Convert_Celsius_Fahrenheit0962932697.htm
http://www.ansleydevore.blogspot.com/ (hahah... since you used mine, I can use yours... that last problem.... whewww!!!!)
1. Thermometers measure temperature by telling how fast the particles inside of the object are moving. Thermometers use mercury, which expands when it gets hot and gets smaller when it cools down.
2. Kelvin and Celsius scales are the same because, although they have different zero points, they still have the same intervals, or degrees. They are different because they are on different scales (288 degrees Kelvin = 15 degrees Celsius = 59 degrees Fahrenheit.) Also, Kelvin isn't really a "degree" of temperature, like Fahrenheit and Celsius.
3. 5 degrees Celsius = 41 degrees Fahrenheit
4. 860 degrees Fahrenheit = 460 degrees Celsius
5. The temperature needs to be raised by 209,000 K.
Cites:
http://wiki.answers.com/Q/How_do_thermometers_measure_temperature
http://en.wikipedia.org/wiki/Temperature_measurement#Comparison_of_temperature_scales
http://wiki.answers.com/Q/How_are_the_three_temperature_scales_alike
http://geography.about.com/c/ht/00/07/How_Convert_Celsius_Fahrenheit0962932697.htm
http://www.ansleydevore.blogspot.com/ (hahah... since you used mine, I can use yours... that last problem.... whewww!!!!)
Test 9 ~ Objective 1
Objective 1:
1. The three common temperature scales are Fahrenheit, Celsius, and Kelvin.
2. Thermal energy is ALL of the energy of the particles that make up an object. Temperature is the measure of how fast or slow particles are moving inside of an object. Heat is the transfer of thermal energy from different objects. Thermal energy is related to heat because heat measures the transfer of thermal energy. Thermal energy relates to temperature because temperature measures how much thermal energy a particle has.
3. Specific heat is the amount of heat that is needed to raise the temperature of an object. An example of something that has a high specific heat is water, because, since it has such strong hydrogen bonds, it needs more energy (heat) to spread the hydrogens out.
4. An ice cube melts in your hand because it is very cold and its particles aren't moving very fast and, when it is put in a human's hand (has heat because of our body heat), it causes the ice cube to get warmer, and it reacts to heating up by melting.
5. One reason some objects get hotter more quickly than others is their "specific heat capacity," or how much heat is needed to raise the temperature of an object (if it has a high specific heat capacity, then it needs more energy to raise its temperature.) Another reason is how much moisture is in an object. For example, you can put some plastic dishes in the microwave because they are made with less water and moisture, so they have a higher specific heat (so they can stay in the microwave longer without getting too hot.)
Cites:
http://abyss.uoregon.edu/~js/glossary/temperature_scale.html
http://www.bristolaggie.mec.edu/school/Velozo/Chapter6.htm
http://wiki.answers.com/Q/Why_does_water_have_a_high_and_specific_heat_capacity
http://answers.yahoo.com/question/index?qid=20100125182103AAM1DPs
1. The three common temperature scales are Fahrenheit, Celsius, and Kelvin.
2. Thermal energy is ALL of the energy of the particles that make up an object. Temperature is the measure of how fast or slow particles are moving inside of an object. Heat is the transfer of thermal energy from different objects. Thermal energy is related to heat because heat measures the transfer of thermal energy. Thermal energy relates to temperature because temperature measures how much thermal energy a particle has.
3. Specific heat is the amount of heat that is needed to raise the temperature of an object. An example of something that has a high specific heat is water, because, since it has such strong hydrogen bonds, it needs more energy (heat) to spread the hydrogens out.
4. An ice cube melts in your hand because it is very cold and its particles aren't moving very fast and, when it is put in a human's hand (has heat because of our body heat), it causes the ice cube to get warmer, and it reacts to heating up by melting.
5. One reason some objects get hotter more quickly than others is their "specific heat capacity," or how much heat is needed to raise the temperature of an object (if it has a high specific heat capacity, then it needs more energy to raise its temperature.) Another reason is how much moisture is in an object. For example, you can put some plastic dishes in the microwave because they are made with less water and moisture, so they have a higher specific heat (so they can stay in the microwave longer without getting too hot.)
Cites:
http://abyss.uoregon.edu/~js/glossary/temperature_scale.html
http://www.bristolaggie.mec.edu/school/Velozo/Chapter6.htm
http://wiki.answers.com/Q/Why_does_water_have_a_high_and_specific_heat_capacity
http://answers.yahoo.com/question/index?qid=20100125182103AAM1DPs
Wednesday, November 10, 2010
Test 8 ~ Objective 6
Objective 6:
1. Charles' Law is a gas law that explains how and why gases get bigger, or "expand" when they are heated.
2. As the temperature of gas increases, the gas molecules move more quickly (because they get more energy in the form of heat.)
3. The first person to fly in a hydrogen balloon was Jacque Charles. The Robert brothers built it for him (Charles invented it), and Charles flew in it.
4. After Charles' first flight in the balloon (it got about fifteen miles outside of Paris, where it took off), it got ripped up by local townspeople because they were scared of it.
5. In the formula below, k is constant (same for all gases and constant pressures). This means that the pressure has to stay the same, and temperature and volume can change
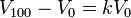
Cites:
http://en.wikipedia.org/wiki/Charles's_law
http://en.wikipedia.org/wiki/Robert_brothers
1. Charles' Law is a gas law that explains how and why gases get bigger, or "expand" when they are heated.
2. As the temperature of gas increases, the gas molecules move more quickly (because they get more energy in the form of heat.)
3. The first person to fly in a hydrogen balloon was Jacque Charles. The Robert brothers built it for him (Charles invented it), and Charles flew in it.
4. After Charles' first flight in the balloon (it got about fifteen miles outside of Paris, where it took off), it got ripped up by local townspeople because they were scared of it.
5. In the formula below, k is constant (same for all gases and constant pressures). This means that the pressure has to stay the same, and temperature and volume can change
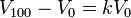
Cites:
http://en.wikipedia.org/wiki/Charles's_law
http://en.wikipedia.org/wiki/Robert_brothers
Monday, November 8, 2010
Test 8 ~ Objective 5
Objective 5:
1. The relationship described in Boyle's Law is between the volume and the pressure of a gas.
2. Scientists only HALF fill high-altitude balloons because as the altitude increases, the pressure decreases. This causes the balloon to get bigger (because volume and pressure are related *when pressure decreases, volume increases*), so if you fill it up all the way, when it expands it will pop.
3. The formula for Boyle's Law is: PV=k. P is pressure of the system, V is volume of the gas, and k is a constant value (of the pressure and volume.)
4. Boyle's Law is useful for physicians because, when they put patients on oxygen (like on the tanks), then they can see how much is in tank so they know when to refill it. They find this out by multiplying the pressure of the oxygen tank by the volume of the tank and find out how much there is / needs to be.
5. Scuba divers use Boyle's Law in the same way that doctors do. When they go underwater, they carry oxygen tanks. They use Boyle's Law to see how much oxygen they have / need.
Citations:
http://en.wikipedia.org/wiki/Boyle's_law
http://wiki.answers.com/Q/Why_do_most_helium_filled_balloons_burst_as_they_ascend_to_high_altitudes
http://wiki.answers.com/Q/What_is_the_formula_of_Boyle's_law
1. The relationship described in Boyle's Law is between the volume and the pressure of a gas.
2. Scientists only HALF fill high-altitude balloons because as the altitude increases, the pressure decreases. This causes the balloon to get bigger (because volume and pressure are related *when pressure decreases, volume increases*), so if you fill it up all the way, when it expands it will pop.
3. The formula for Boyle's Law is: PV=k. P is pressure of the system, V is volume of the gas, and k is a constant value (of the pressure and volume.)
4. Boyle's Law is useful for physicians because, when they put patients on oxygen (like on the tanks), then they can see how much is in tank so they know when to refill it. They find this out by multiplying the pressure of the oxygen tank by the volume of the tank and find out how much there is / needs to be.
5. Scuba divers use Boyle's Law in the same way that doctors do. When they go underwater, they carry oxygen tanks. They use Boyle's Law to see how much oxygen they have / need.
Citations:
http://en.wikipedia.org/wiki/Boyle's_law
http://wiki.answers.com/Q/Why_do_most_helium_filled_balloons_burst_as_they_ascend_to_high_altitudes
http://wiki.answers.com/Q/What_is_the_formula_of_Boyle's_law
Sunday, November 7, 2010
Test 8 ~ Objective 4
Objective 4:
1. The thermal energy (heat) of objects at a warmer temperature is a lot warmer than the thermal energy of objects at a cooler temperature. This is because particles in these objects react to heat by jumping around, or getting excited, which causes an object to be warm. If there is no heat, the particles aren't jumping around, so there is no heat produced.
2. Ice cream can melt from temperature. This is because, since it is so hot outside, the ice cream is absorbing a lot of energy from the sun, which causes it to melt because of the question above (#1.) Another reason why ice cream melts is the ingredients inside of it, which includes fat. Non-fat ice cream will melt more slowly than regular ice cream because, since there is less fat, there is less water. In non-fat ice cream, there is more water, which means it will have to absorb more energy in order to melt.
3. The melting point of particles has to do with the vibrating particles because when particles in an object absorb heat, or energy, they start to move around (vibrate), and this causes them to melt. The more particles vibrate, the faster they melt.
4. Condensation occurs when humid air, or air that is "saturated with moisture" cools down very quickly. This causes the water to make little droplets. An example of this is when there is condensation on a cup with cold liquid inside of it.
5. Sublimation is when a solid goes straight to a gas, without being a liquid first. Sublimation happens when there is low humidity and the temperature is just below freezing.
Cites:
http://www.post-gazette.com/pg/09187/981622-115.stm
http://wiki.answers.com/Q/Why_does_condensation_occur
http://www.newton.dep.anl.gov/askasci/chem00/chem00402.htm
1. The thermal energy (heat) of objects at a warmer temperature is a lot warmer than the thermal energy of objects at a cooler temperature. This is because particles in these objects react to heat by jumping around, or getting excited, which causes an object to be warm. If there is no heat, the particles aren't jumping around, so there is no heat produced.
2. Ice cream can melt from temperature. This is because, since it is so hot outside, the ice cream is absorbing a lot of energy from the sun, which causes it to melt because of the question above (#1.) Another reason why ice cream melts is the ingredients inside of it, which includes fat. Non-fat ice cream will melt more slowly than regular ice cream because, since there is less fat, there is less water. In non-fat ice cream, there is more water, which means it will have to absorb more energy in order to melt.
3. The melting point of particles has to do with the vibrating particles because when particles in an object absorb heat, or energy, they start to move around (vibrate), and this causes them to melt. The more particles vibrate, the faster they melt.
4. Condensation occurs when humid air, or air that is "saturated with moisture" cools down very quickly. This causes the water to make little droplets. An example of this is when there is condensation on a cup with cold liquid inside of it.
5. Sublimation is when a solid goes straight to a gas, without being a liquid first. Sublimation happens when there is low humidity and the temperature is just below freezing.
Cites:
http://www.post-gazette.com/pg/09187/981622-115.stm
http://wiki.answers.com/Q/Why_does_condensation_occur
http://www.newton.dep.anl.gov/askasci/chem00/chem00402.htm
Thursday, November 4, 2010
Test 8 ~ Objective 3
Objective 3:
1. Some of the forms of energy related to changes in matter are chemical energy (because it makes a chemical reaction, which can make a different substance, such as plasma), and thermal energy.
2. A rolling ball has kinetic energy (because it is in motion.)
3. A bowling ball sitting still has potential energy (because it is sitting in place.)
4. Electromagnetic energy is energy of or from radiation. It can transmit energy in the form of waves from one place to another. The stuff that it transmits can be in the form of heat, light, or anything else.
5. The energy of electrons moving from one place to another is called electric energy. An example is this is charging a computer. The electrons go from a power line, into a wire that connects our school, through the wall and into the power cord, and then finally (when you plug it in) the electrons go into your computer.
Cites:
http://www.buzzle.com/articles/what-is-electromagnetic-energy.html
http://www.historyoftheuniverse.com/chemener.html
1. Some of the forms of energy related to changes in matter are chemical energy (because it makes a chemical reaction, which can make a different substance, such as plasma), and thermal energy.
2. A rolling ball has kinetic energy (because it is in motion.)
3. A bowling ball sitting still has potential energy (because it is sitting in place.)
4. Electromagnetic energy is energy of or from radiation. It can transmit energy in the form of waves from one place to another. The stuff that it transmits can be in the form of heat, light, or anything else.
5. The energy of electrons moving from one place to another is called electric energy. An example is this is charging a computer. The electrons go from a power line, into a wire that connects our school, through the wall and into the power cord, and then finally (when you plug it in) the electrons go into your computer.
Cites:
http://www.buzzle.com/articles/what-is-electromagnetic-energy.html
http://www.historyoftheuniverse.com/chemener.html
Wednesday, November 3, 2010
Test 8 ~ Objective 2
Objective 2:
1. The main difference between a chemical change and a physical change is that in a physical change, no new substances are formed, and in a chemical change, a new substance is formed. An example of a physical change is freezing water (you can melt it and it still is water.) An example of a chemical change is burning something because the solid that you burn breaks down (because of the heat- SUBLIMATION!!) into many other elements, such as carbon dioxide.
2. The four ways that chemical changes can occur are a solid is formed, a color change happens, a gas is produced (you can tell by bubbles forming), and light, heat, or sound is produced.
3. The Law of Conservation of Mass basically says that mass of an isolated system (which cannot exchange heat, work, or matter with its surroundings) cannot be created or destroyed, but it can be moved around. The law was created by Empedocles, who an ancient Greek philosopher. His idea was "Nothing comes from nothing."
4. Temperature and thermal energy are different because temperature isn't actually energy, but it is a number that is related to energy, or heat ( is related to the average kinetic energy of the molecules in a substance). Thermal energy is ACTUAL energy that is measured in energy units and is the measure of energy in a substance.
5. An exothermic reaction is a reaction that releases energy in the form of heat. An example of this is elephant toothpaste, which reacted by getting very hot. An endothermic (absorbs heat from surroundings) reaction is when an object gets cold (because it is taking in the heat, not releasing it.) An example of this is the experiment that Gabby and I did because the acentric acid and the baking soda reacted to each other by getting very cold.
Cites:
http://www.physlink.com/education/askexperts/ae244.cfm
http://en.wikipedia.org/wiki/Conservation_of_mass
http://zonalandeducation.com/mstm/physics/mechanics/energy/heatAndTemperature/heatAndTemperature.html
http://en.wikipedia.org/wiki/Endothermic_reaction
http://wiki.answers.com/Q/Four_ways_you_can_tell_a_chemical_change_has_taken_place
1. The main difference between a chemical change and a physical change is that in a physical change, no new substances are formed, and in a chemical change, a new substance is formed. An example of a physical change is freezing water (you can melt it and it still is water.) An example of a chemical change is burning something because the solid that you burn breaks down (because of the heat- SUBLIMATION!!) into many other elements, such as carbon dioxide.
2. The four ways that chemical changes can occur are a solid is formed, a color change happens, a gas is produced (you can tell by bubbles forming), and light, heat, or sound is produced.
3. The Law of Conservation of Mass basically says that mass of an isolated system (which cannot exchange heat, work, or matter with its surroundings) cannot be created or destroyed, but it can be moved around. The law was created by Empedocles, who an ancient Greek philosopher. His idea was "Nothing comes from nothing."
4. Temperature and thermal energy are different because temperature isn't actually energy, but it is a number that is related to energy, or heat ( is related to the average kinetic energy of the molecules in a substance). Thermal energy is ACTUAL energy that is measured in energy units and is the measure of energy in a substance.
5. An exothermic reaction is a reaction that releases energy in the form of heat. An example of this is elephant toothpaste, which reacted by getting very hot. An endothermic (absorbs heat from surroundings) reaction is when an object gets cold (because it is taking in the heat, not releasing it.) An example of this is the experiment that Gabby and I did because the acentric acid and the baking soda reacted to each other by getting very cold.
Cites:
http://www.physlink.com/education/askexperts/ae244.cfm
http://en.wikipedia.org/wiki/Conservation_of_mass
http://zonalandeducation.com/mstm/physics/mechanics/energy/heatAndTemperature/heatAndTemperature.html
http://en.wikipedia.org/wiki/Endothermic_reaction
http://wiki.answers.com/Q/Four_ways_you_can_tell_a_chemical_change_has_taken_place
Tuesday, November 2, 2010
Test 8 ~ Objective 1
Objective 1:
1. Mass is more useful than weight in measuring matter because mass is always the same, and weight changes depending on gravity. Since scientist don't want to get different answers, they use mass because it never changes, or it is a constant. I think (I am not sure) that an example of this is when you are on the moon, you have a lighter weight (because there is less gravity) than when you are on earth, but you have the same mass in both places.
2. The volume of the plastic box is 619.65 cm3 (centimeters cubed.)
3. The unit of measurement for density is the size (ex: g) and the unit of volume (cm3 *centimeters cubed*.) For example, is something that was 2 grams had a volume of 12 cm3 (centimeters cubed), you would put 2 g/ 12 cm3 (centimeters cubed.)
4. The formula for finding density is mass divided by volume, or mass over volume.
5. The formula for finding volume is length multiplied by width multiplied by height (l*w*h)
Cites:
http://wiki.answers.com/Q/Why_is_mass_more_useful_than_weight_for_measuring_matter
http://en.wikipedia.org/wiki/Density
http://en.wikipedia.org/wiki/Volume
1. Mass is more useful than weight in measuring matter because mass is always the same, and weight changes depending on gravity. Since scientist don't want to get different answers, they use mass because it never changes, or it is a constant. I think (I am not sure) that an example of this is when you are on the moon, you have a lighter weight (because there is less gravity) than when you are on earth, but you have the same mass in both places.
2. The volume of the plastic box is 619.65 cm3 (centimeters cubed.)
3. The unit of measurement for density is the size (ex: g) and the unit of volume (cm3 *centimeters cubed*.) For example, is something that was 2 grams had a volume of 12 cm3 (centimeters cubed), you would put 2 g/ 12 cm3 (centimeters cubed.)
4. The formula for finding density is mass divided by volume, or mass over volume.
5. The formula for finding volume is length multiplied by width multiplied by height (l*w*h)
Cites:
http://wiki.answers.com/Q/Why_is_mass_more_useful_than_weight_for_measuring_matter
http://en.wikipedia.org/wiki/Density
http://en.wikipedia.org/wiki/Volume
Friday, October 22, 2010
Endothermic Reaction Video (almost)
Gabby and I did our reaction today. We made a video...but someone kindly deleted it for us! haha! O well...we will just explain it.
Our experiment called for 25ml of acetic acid and 15g of baking soda.... we didn't have that much acid, so we divided everything by 5 so we didn't have to use as much, but it was still balanced. Since we didn't use as much ingredients as the experiment called for, our results weren't very dramatic, but it still worked. The temperature went down 3-4 degrees Celsius.... and ended up being at about 18 degrees Celsius.
When we mixed everything together, we saw a few bubbles forming (a sign of a chemical reaction) and then it got colder. Again, there would have been more bubbles if we used as much ingredients as the experiment called for. Also, the acid smelled TERRIBLE!!!!! You would have seen it on our video, but apparently someone thought theirs was better :) hahaha.... just kidding!!!!
That is about it.... have a good weekend!!
Our experiment called for 25ml of acetic acid and 15g of baking soda.... we didn't have that much acid, so we divided everything by 5 so we didn't have to use as much, but it was still balanced. Since we didn't use as much ingredients as the experiment called for, our results weren't very dramatic, but it still worked. The temperature went down 3-4 degrees Celsius.... and ended up being at about 18 degrees Celsius.
When we mixed everything together, we saw a few bubbles forming (a sign of a chemical reaction) and then it got colder. Again, there would have been more bubbles if we used as much ingredients as the experiment called for. Also, the acid smelled TERRIBLE!!!!! You would have seen it on our video, but apparently someone thought theirs was better :) hahaha.... just kidding!!!!
That is about it.... have a good weekend!!
Wednesday, October 20, 2010
Endothermic Reaction
Hey everybody!! Gabby and I are doing endothermic reactions for Test 6. We will have a video posted soon of our actual experiment and all of our materials and results.
Endothermic: "within-heating" --> describes a process or reaction in which the system absorbs energy from the surroundings in the form of heat (takes in heat and doesn't produce it... so it is a cold reaction)
Materials
~ 25 ml of acetic acid
~15 g of baking soda
~ a styrofoam cup
~ a thermometer
~ stirring rod
Endothermic: "within-heating" --> describes a process or reaction in which the system absorbs energy from the surroundings in the form of heat (takes in heat and doesn't produce it... so it is a cold reaction)
Materials
~ 25 ml of acetic acid
~15 g of baking soda
~ a styrofoam cup
~ a thermometer
~ stirring rod
Thursday, September 30, 2010
Breaking Down Sugar~ Lab
Hey everybody! Gabby and I finished our breaking down sugar down and made a video of the results.
Breaking Down Sugar~Lab
Breaking Down Sugar~Lab
Thursday, September 16, 2010
Test 1~Part 3
Hey everybody!!! This picture is the final part of Test 1!!! Gabby and I made this on Sunday. The black golf balls represent protons, and the white ones represent neutrons. The marbles around it represent the electrons, orbiting around the nucleus. Although the hula hoops don't represent where the electrons necessarily are, they represent where they could be. Hope you all like it!
Here is our model of Boron!!!!!
Here is our model of Boron!!!!!
Monday, September 13, 2010
Test 1~Part 2
Here is my paper that I wrote about atoms!
Atom Paper
The cites I used were:
http://www.answers.com/topic/antiproton
http://www.wikipedia.org/
all material covered in Mr. Harrelson's class
Atom Paper
The cites I used were:
http://www.answers.com/topic/antiproton
http://www.wikipedia.org/
all material covered in Mr. Harrelson's class
Sunday, September 12, 2010
Test 1~Part 1
Hey everybody! Here is my the test I made for Test 1. It would be great if anyone wanted to take it (I promise there are no essays!)
Atomic Model Test
Atomic Model Test
Tuesday, September 7, 2010
Extra Credit
Ok, sorry.... I haven't learned how to edit my post yet, so I accidentally hit publish (again.) Anyway, this picture is of the crystalline in the compound.

Here is another picture of the compound itself....
I got all of my information off of Wikipedia :)
Here is another picture of the compound itself....
I got all of my information off of Wikipedia :)
Extra Credit
Aluminum Oxide is an inorganic compound that has a common name of alumina, and is mostly used in the production of aluminum. It is an amphoteric oxide, which means that it can react to either an acid or a base. Corundum is a crystalline form of this compound, which basically means that the atoms, molecules, or ions are arranged in an organized way.
This picture is of it's crystalline solid pattern-->
This picture is of it's crystalline solid pattern-->
Monday, September 6, 2010
Parts of Atoms II
Sorry about the last post... I accidentally hit "Publish." Ok, so there is a very LONG video posted, and I completely understand if you don't want to watch it... so here is a quick recap of everything on it. :)
- protons=positive charge, neutrons=neutral charge, and electrons=negative charge
- protons and neutrons are in the nucleus, and electrons orbit the nucleus
- protons are made of quarks ( Up, Up, Down)
- neutrons are make of quarks (Down, Down, Up)
- six types of quarks = Up, Down, Top, Bottom, Charm, Strange
- electrons are EXTREMELY small
- amu = atomic mass units (electrons= 1/1836, protons and neutrons= 1)
- atomic number is found at the top of the square (number of protons)
- electrons don't have quarks and gluons
- electrons are used for electricity
- gluons hold quarks together
- model of what an atom really looks like and what it looks like for our purposes
this is a model of what an atom looks like for our purposes.... is this dumbed down or what??????? hahaha
Hope this helps everyone! Happy Labor Day!
Saturday, September 4, 2010
Tuesday, August 31, 2010
Democritus vs. Shroedinger
Democritus and Shroedinger were both very well known scientists in their time, and both did a lot of work with atoms and matter...., but they had very different methods when it came to the use of the scientific method.
Scientific Method (from http://www.sciencebuddies.org/science-fair-projects/project_scientific_method.shtml)
~Ask a Question
~Do Background Research
~Construct a Hypothesis
~Test Your Hypothesis by Doing an Experiment
~Analyze Your Data and Draw a Conclusion
~Communicate Your Results
Ok, so a little bit about Democritus...
He was an ancient Greek philosopher who lived around 430 BCE. He had an idea while he was tearing a piece of bread that there would be a piece that couldn't be torn any smaller. His idea was that the definition of an atom was "uncuttable." He thought that, for example, if you cut a tree into several different pieces, there would eventually be a piece of tree that couldn't be cut any more (he was later proved wrong because the tree would just be divided into millions of atoms.) He also thought that the strength of an object had to do with the atoms and how they were shaped/arranged. He thought that atomos (what he named atoms) were everywhere and there were tons of them.
And now a little about Shroedinger...
Shroedinger was a theoretical physicist who was from Austria. He was famous for laying the foundation for "quantam physics." Another famous thing that he did was an experiment where he put a cat in a box with poison in it and asked if the cat was dead or not.... it was a trick question because you didn't know for sure, but could only hypothesize (it is still being debated today). He stressed the importance to not only find out how electrons move, but to figure out where we could find them.
Both Democritus and Shroedinger.....
Democritus didn't exactly use the scientific method in his idea/hypothesis. For example, he did have a question about the smallest piece of bread, but he didn't do an experiment or background research. Instead of doing research and an experiment, he assumed that there was a piece of bread that couldn't be cut any smaller, and that atoms were uncuttable. Shroedinger, on the other hand, did use the scientific method. He had a question, carried it out with an experiment (Shroedinger's cat), and then analyzed the data. Part of the reason why Democritus didn't do an experiment was because he lived in a very early time period, and maybe didn't have the right materials. Shroedinger also had many other discoveries by other scientists before his time (e.g.- Thomson, Dalton, Rutherford) and he had all of that research to base his experiment on.
All of this information was found on Wikipedia and Mr. Harrelson's slideshow :)
Scientific Method (from http://www.sciencebuddies.org/science-fair-projects/project_scientific_method.shtml)
~Ask a Question
~Do Background Research
~Construct a Hypothesis
~Test Your Hypothesis by Doing an Experiment
~Analyze Your Data and Draw a Conclusion
~Communicate Your Results
Ok, so a little bit about Democritus...
He was an ancient Greek philosopher who lived around 430 BCE. He had an idea while he was tearing a piece of bread that there would be a piece that couldn't be torn any smaller. His idea was that the definition of an atom was "uncuttable." He thought that, for example, if you cut a tree into several different pieces, there would eventually be a piece of tree that couldn't be cut any more (he was later proved wrong because the tree would just be divided into millions of atoms.) He also thought that the strength of an object had to do with the atoms and how they were shaped/arranged. He thought that atomos (what he named atoms) were everywhere and there were tons of them.
And now a little about Shroedinger...
Shroedinger was a theoretical physicist who was from Austria. He was famous for laying the foundation for "quantam physics." Another famous thing that he did was an experiment where he put a cat in a box with poison in it and asked if the cat was dead or not.... it was a trick question because you didn't know for sure, but could only hypothesize (it is still being debated today). He stressed the importance to not only find out how electrons move, but to figure out where we could find them.
Both Democritus and Shroedinger.....
Democritus didn't exactly use the scientific method in his idea/hypothesis. For example, he did have a question about the smallest piece of bread, but he didn't do an experiment or background research. Instead of doing research and an experiment, he assumed that there was a piece of bread that couldn't be cut any smaller, and that atoms were uncuttable. Shroedinger, on the other hand, did use the scientific method. He had a question, carried it out with an experiment (Shroedinger's cat), and then analyzed the data. Part of the reason why Democritus didn't do an experiment was because he lived in a very early time period, and maybe didn't have the right materials. Shroedinger also had many other discoveries by other scientists before his time (e.g.- Thomson, Dalton, Rutherford) and he had all of that research to base his experiment on.
All of this information was found on Wikipedia and Mr. Harrelson's slideshow :)
Sunday, August 29, 2010
Extra Credit
Okay.... so here is my attempt for the extra credit!
Oxygen~
protons: 8
electrons: 8
neutrons: 7 ( I think:)
atomic number: 8
atomic mass: 15.9994
Oxygen~
protons: 8
electrons: 8
neutrons: 7 ( I think:)
atomic number: 8
atomic mass: 15.9994
Wednesday, August 25, 2010
First Day
Hey Mr. Harrelson... I explained to my parents that I had to start a blog for science, but they didn't exactly understand the concept of a "science blog" and thought that I should put some Mos Def quotes on this thing! I told them that I didn't think that was the idea.... just to do my homework, so I'll spare you the quotes and just send you a link to one of his videos. I know that you will be getting a lot of interesting science links, so I thought this would be different.
Mos Def for President
Mos Def for President
Subscribe to:
Comments (Atom)

